Does Flour Dissolve In Water (And Why)? The Science Explained
Have you ever been baking and started to wonder why the flour you might be using forms a kind of paste when placed in water? After recently observing this phenomenon, I decided to dive deeper into the science behind it.
Generally, flour does not dissolve in water as it consists of starch granules, proteins and lipids that are all insoluble in water due to their molecular structure. Instead of dissolving in water, flour will absorb water to form a sticky suspension.
Something important to keep in mind is that the solubility of flour in water will depend on multiple different factors, such as the type of flour used as well as water temperature. These factors are discussed later in the article.
What Is Flour Actually Made Of?
Flour is typically made of different kinds of grains through a grinding process of some sort. These grains are made into a powder that can then be used for lots of different foods and baking products.
However, I want to dive a bit deeper into the actual molecular structure of flour, as that is what best explains its water solubility.
Chemically, flour is made of a mixture of different proteins, saccharide molecules (the main one being starch, which, furthermore, is a mixture of amylopectin and amylose molecules) and a small percentage of lipids. The component ratios will vary a bit depending on the type of flour.
For reference, the composition of wheat flour is 10-12 % protein, 70-75 % starch, 2-3 % other polysaccharides and 2% lipids, with the rest being moisture or water. This is, according to an analysis conducted by Henan University of Technology (published in 2019).
| Compound | Percentage |
|---|---|
| Proteins | 10-12 % |
| Starch | 70-75 % |
| Polysaccharides (other than starch) | 2-3 % |
| Lipids | 2 % |
| Water/moisture | 8-16 % |
These molecules and components all have their own properties in terms of structure, which also further explains their water solubility.
For instance, depicted below is the structure of a starch molecule, which consists of chains of glucose molecules joined together through their hydroxyl groups (the little OH-parts) reacting with each other.
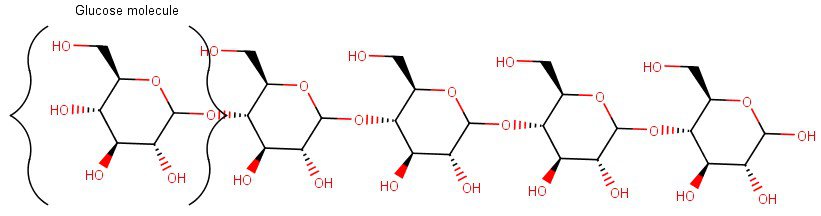
Of course, these drawings like the one above are just simplistic 2-dimensional sketches of the starch molecule.
In reality, multiple of these glucose chains (which have either an amylopectin or an amylose structure, depending on how the glucose chains are branched out) can also join together from all directions with hydrogen bonds between the chains to form a crystalline (helix-shaped) structure of a starch granule.
Why Is Flour Not Soluble In Water?
The helical structures found in starch molecules mentioned above play a big role in the water solubility of flour.
In short, flour is not soluble in water as it is mostly made of starch that has a tightly packed helical structure. This prevents flour from bonding with water molecules, thus making it insoluble in water. Flour also contains the protein called gliadin and some lipids, both of which are insoluble in water.
Sometimes you might hear that the molecules in flour (such as starch) are just too big to be dissolved in water, which would explain its water-insolubility. This, however, is not really true.
Usually, molecular size does not play much of an important role in water solubility – molecular structure is a much more important factor to consider in regards to this.
In the case of starch, it is made of amylopectin and amylose molecules – in fact, amylopectin is a larger molecule, yet it is still more water-soluble than amylose.
This comes down to the fact that amylose has a kind of helical structure (similar to DNA), which is a result of all the atoms aligning in the most low-energy way possible and therefore, they form this helix shape.
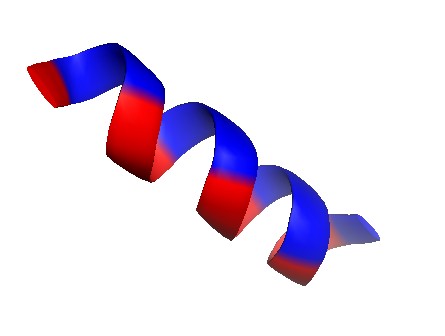
This helical structure is too tightly packed for water molecules to get within its structure, thus making amylose generally insoluble in water.
Amylopectin, on the other hand, has a more linear structure that is also very branched out. This makes it easy for water molecules to form hydrogen bonds with the hydroxyl (OH) groups found in the amylopectin molecule – which further makes them water soluble.
So, the bottom line is that molecular structure is the deciding factor in water solubility, not molecule size.
For more on the solubility of amylose and amylopectin, here’s a cool paper on the topic from the Journal of Chemical Education.
The interesting thing, however, is that in starch, both amylose and amylopectin will take on a helical structure that prevents water molecules from getting within the structure. This is the precise reason why starch is insoluble in water.
This also explains why flour has a fairly low water solubility as it is largely made of starch. Flour also contains proteins like gliadin, which are insoluble in water. Small amounts of fatty acids and lipids are found in flour too, which also are water-insoluble.
What Happens When Flour Is Mixed With Water?
We’ve all probably seen the making of bread at some point. But have you ever actually wondered what happens when flour is mixed with water, in terms of the chemistry behind it?
When mixed with water, the proteins in flour, gliadin and glutenin, will begin to react and bond to form a protein network of gluten, which then turns the flour into dough. If heated at the same time, the starch granules in flour will break down and absorb water, causing swelling.
The gluten structure made of these gliadin and glutenin bonds is actually what is responsible for the swelling of dough in the baking process.
This is because the gluten network in the dough that forms when flour and water are mixed is quite a tight structure. When yeast is then added, it will produce carbon dioxide gas that is unable to escape the gluten structure, making the entire dough swell – something you might be well aware of already!
In fact, there is actually quite a lot of chemistry going on in the flour-water mix. This video explains some of that pretty well:
Which Flour Absorbs More Water (And Why)?
The water absorption of flour depends on many factors, however, there is still some baseline rules for which flours will absorb more water.
Generally, flours high in gluten-forming proteins (hard flours) will absorb more water than low protein flours (soft flours). Whole wheat flour will also absorb more water than white flour or all-purpose flour as it contains more water-absorbing substances, such as fiber.
The reason for this is that – as explained above – gluten will absorb water, which is why flours higher in gluten-forming proteins generally absorb more water. An example of this is bread flour, which is quite high in proteins that form an elastic water-absorbing gluten network.
The amount of starch in the flour at hand will also play a role in its water absorption. This is because as the starch granules begin to break, they will also begin absorbing water as the water molecules are now able to get within the starch molecule structure.
Something to also keep in mind is that the storage conditions for a given flour will also play a role in its water absorption. Generally, flour stored in a dry environment will absorb more water as it has lost some of its moisture content due to the dry storage conditions.