Can Diamonds Melt Or Burn (And How)? The Science Explained
The old saying goes that diamonds are forever, but are they really? Diamonds are certainly one of the strongest substances in the world, but can they be burned or even melted?
In short, diamonds will burn at around 900 °C given that there is enough oxygen for the burning process. Diamonds can also melt given a temperature of at least 4500 °C and a pressure of 100 000 bar, which is about 100 000 times higher than the standard atmospheric pressure.
There is, however, more to the story. These fairly specific burning and melting conditions can be explained by the chemical and physical properties of diamonds, which can then explain why, for example there may even exist liquid diamond oceans on Neptune and Uranus.
Here is a table of the burning and melting points of a diamond:
| Burning point | Melting point | |
|---|---|---|
| Temperature | 900 °C | 4500 °C |
| Pressure | 1 bar (1 atm) | 100 000 bar (100 000 atm) |
Later in the article I’m going to explain exactly how these burning and melting processes work as well as talk about whether it’s even possible to actually melt a diamond.
What Is The Burning Point Of A Diamond?
You’re most likely familiar with the idea that diamonds require quite harsh conditions to even form in the first place. This also works the other way around. But what exactly are the burning conditions for a diamond?
The temperature required for a diamond to begin burning is roughly 900 °C (equivalent to 1650 Fahrenheit or 1200 kelvin) at atmospheric pressure. Sufficient oxygen is also required for the burning process.
The scientific definition for burning (or more accurately, combustion) is a chemical reaction with oxygen that releases energy into the surrounding environment.
Diamonds are no exceptions for this definition, and in fact combustion does happen for diamonds as well, since they are basically made of carbon.
When a diamond is heated enough and in contact with oxygen, it will burn and start to react with the oxygen to form either carbon monoxide or carbon dioxide depending on how much oxygen there is available:
C+\frac{1}{2}O_2\ \rightarrow\ CO\\C+O_2\ \rightarrow\ CO_2This is really no different than the burning of, for example regular coal, which would have the exact same chemical equation (assuming that the coal is made up entirely of carbon).
If oxygen is NOT available, no burning reaction can happen. In these kinds of conditions, when heated to a high temperature, the diamond will instead turn into graphite.
This is due to the fact that the high temperature will break the diamond structure, and as there is no oxygen to react with, the diamond will change its structure to graphite as it is a more stable and favorable form in normal, low pressure conditions.
What Does A Burning Diamond Look Like?
The burning of a diamond sadly isn’t really as fancy as it should considering it’s an actual diamond. Although, it does shine quite brightly, it doesn’t quite light on fire like we typically think of something burning.
Anyway, here’s a cool video of a burning diamond:
Are There Actual Practical Ways To Burn a Diamond?
The next question you might have is that how would you actually burn a diamond? What would be the most practical way to do it?
Most likely the easiest way to burn a diamond would be to dip it into some liquid oxygen. This would ensure that there is at least sufficient oxygen for the burning to happen.
The problem is that for oxygen to exist as a liquid, it has to have a temperature of below -183 °C (in atmospheric pressure). This means that the temperature may generally be too low to actually start breaking the bonds within the diamond structure.
Therefore, a more efficient way might be to first heat the diamond up to a very high temperature (with a torch or something) and then drop it into some liquid oxygen, as seen in the video above.
What Is The Melting Point Of A Diamond?
The idea of a molten diamond may seem quite odd as you probably have never seen or even heard of it. This is because the conditions for melting diamonds are so extreme and also very rare.
Essentially, a diamond will melt at a minimum temperature of about 4500 °C (a little over 4700 K) and a pressure of 100 000 bar (which is about 10 GPa or 100 000 atm).
Also, the concept of a “liquid diamond” is also a bit questionable as diamonds are generally defined as just carbon that is structured in a specific way. So, there is definitely an argument to be made for whether a molten diamond is even a diamond anymore or just simply liquid carbon.
Anyway, it is still possible to turn a diamond into a molten form under specific conditions.
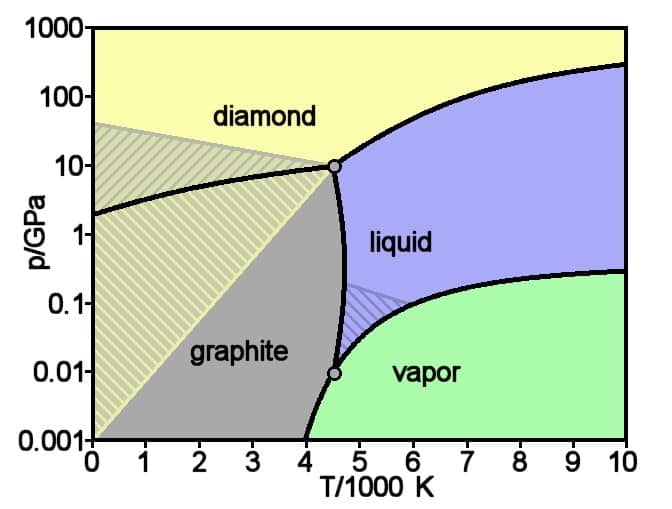
The above picture is the phase diagram of carbon, which describes the specific forms (phases) of carbon and the general forming conditions for these phases.
From this diagram, we can pick out the conditions for when a diamond crosses over to the liquid phase (i.e. the melting point), which are:
- Minimum temperature of 4500 °C (around 4700 K).
- Pressure of 100 000 bar (about 10 GPa or 100 000 atm).
The interesting thing about these conditions is that a diamond can not actually melt at normal atmospheric pressure levels, so to do it would definitely require a laboratory of some sorts.
This is also where the problem of melting a diamond occurs. See, the diamond would turn into graphite way before it actually reaches the melting conditions when heated in the absence of oxygen (as explained earlier).
On the other hand, if oxygen is present, the diamond would just burn away before melting.
This is why a high pressure is required as diamond actually becomes a more stable form than graphite in high pressure conditions. Therefore, the diamond would not turn into graphite before melting, given of course that the pressure is sufficiently high.
Can Diamonds Be Melted In Laboratories?
Luckily, the melting of a diamond isn’t just something to theoretically wonder about. Scientists have actually managed to do it in the real world.
The Z machine, a part of the Sandia National Laboratories in Albuquerque, New Mexico, was able to melt diamonds in 2006 by creating insanely high pressures in laboratory conditions (here’s the story from ScienceDaily).
The way diamonds we’re melted was by shooting little metal plates at small pieces of diamond by using extremely strong magnetic fields. The small plates would then strike the diamonds evenly from every direction to create a shockwave equivalent to more than 10 million times the atmospheric pressure.
If you wish to, you can read more about the fascinating Sandia Z machine from the Sandia National Laboratories website.
Can Lava Melt A Diamond?
How interesting would it be to see a diamond melt into liquid when placed in a puddle of lava. But would this actually be possible?
To put it simply, a diamond cannot melt in lava, because the melting point of a diamond is around 4500 °C (at a pressure of 100 kilobars) and lava can only be as hot as about 1200 °C.
Can Diamonds Burn In Lava?
Lava isn’t quite hot enough to melt a diamond, but could a diamond burn in lava instead?
In short, diamonds can burn in lava as the burning temperature of a diamond is about 900 °C and lava can get as hot as 1200 °C. The burning process will also, however, require oxygen.
Can Acid Dissolve Diamonds?
Acids, especially some very corrosive ones, can dissolve many things. But could an acid possibly dissolve a diamond?
In short, acids do not dissolve diamonds because there simply isn’t an acid corrosive enough to destroy the strong carbon crystal structure of a diamond. Some acids may, however, damage diamonds.
Down below is an interesting experiment from The Action Lab, where a diamond is place into a container of piranha solution, an extremely corrosive acid mixture.
Long story short, the diamond barely got dissolved at all and it wasn’t even clear whether the diamond actually lost any mass or if there was simply a slight deviation in the reading of the scale.
Also, if you’re wondering whether acid could dissolve glass and which acids can actually do that (there are certainly acids that can!), I’d recommend reading this article.